Trading accounts
Platforms
News
We provide the latest news
from the world of economics and finance
We provide the latest news from the world of economics and finance
Novartis NVS announced positive top-line results from a late-stage study on Fabhalta (iptacopan) in adult patients with paroxysmal nocturnal hemoglobinuria (PNH).
Fabhalta is an oral, Factor B inhibitor of the alternative complement pathway.
APPULSE-PNH is a phase IIIB multicenter, single-arm, open-label study to evaluate the efficacy and safety of twice-daily oral Fabhalta monotherapy (200mg) in adults with PNH who were switched from anti-C5 therapies (eculizumab or ravulizumab).
Year to date, shares of Novartis have risen 0.7% compared with the industry’s growth of 9.5%.
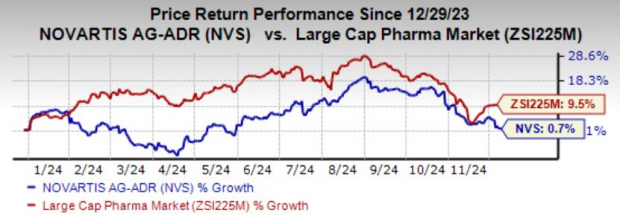
Image Source: Zacks Investment Research
The APPULSE-PNH study enrolled 52 participants who received Fabhalta for 24 weeks.
In the Phase IIIB APPULSE-PNH study, oral Fabhalta improved the average hemoglobin (Hb) level versus baseline in adult patients with PNH who were switched from anti-C5 therapies.
The positive results reinforce Fabhalta's ability to benefit both patients previously treated with anti-C5 therapies studied in the APPULSE-PNH and APPLY-PNH trials and complement-inhibitor naïve patients studied in the APPOINT-PNH trial.
The safety profile of Fabhalta monotherapy was consistent with previously reported data.
Fabhalta obtained FDA approval in December 2023 for the treatment of adults with PNH. It also received the European Medicines Agency’s approval in May 2024 for the treatment of adults with PNH with hemolytic anemia.
We remind investors that the FDA recently granted Fabhalta accelerated approval for reducing proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk of rapid disease progression.
Fabhalta is being evaluated in a broad range of rare kidney diseases, including C3 glomerulopathy, atypical hemolytic uremic syndrome, immune complex membranoproliferative glomerulonephritis and lupus nephritis.
Novartis also announced positive, longer-term results from the phase III ASC4FIRST study on leukemia drug Scemblix (asciminib).
Scemblix demonstrated sustained superior major molecular response (MMR) versus all investigator-selected tyrosine kinase inhibitors (TKIs) and versus imatinib alone. Scemblix met both key secondary endpoints at the end of 96 weeks.
Scemblix showed a clinically relevant 15.1% higher MMR rate versus second-generation TKIs (72.0% versus 56.9%).
NVS is a pure-play innovative medicine company with a focus on core therapeutic areas — cardiovascular, renal and metabolic, immunology, neuroscience and oncology.
Novartis’ performance in the third quarter was impressive, with both earnings and sales beating estimates. The increase in annual guidance was another positive, indicating strong momentum across all key drugs in the upcoming quarters.
Novartis recently increased its sales guidance for the mid-term. The company now expects total sales to witness a compound annual growth rate (CAGR) of 6% during 2023-2028 compared with the previous estimate of 5%.
The rise in guidance was due to the strong momentum of NVS’ key drugs and its upcoming launches.
While organic growth continues to drive business, NVS is focused on strategic bolt-in acquisitions to strengthen its pipeline.
Novartis recently entered into a global license and collaboration agreement with PTC Therapeutics PTCT for the latter’s Huntington's disease candidate to strengthen NVS’ neuroscience pipeline.
Novartis will make an upfront payment of $1.0 billion to PTCT and the latter will also be eligible for up to $1.9 billion in development, regulatory and sales milestones. NVS will also share profits in the United States (40% PTC and 60% Novartis) and pay tiered royalties on ex-US sales.
NVS announced a collaboration with Olema Pharmaceuticals, Inc. OLMA for a breast cancer drug.
Per the terms of the deal, Novartis will provide Olema with its breast cancer drug Kisqali (ribociclib) for the planned phase III OPERA-02 trial of Olema’s palazestrant in combination with ribociclib in ER+/HER2- frontline advanced or metastatic breast cancer.
Novartis currently carries a Zacks Rank #3 (Hold).
A better-ranked stock from the large-cap pharma industry is Pfizer PFE, which currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Over the past 60 days, Pfizer’s earnings estimates have risen from $2.62 to $2.91 per share for 2024, while that for 2025 has increased from $2.84 to $2.91. PFE’s shares have lost 10% year to date.
Pfizer’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 74.50%.
The demand for electricity is growing exponentially. At the same time, we’re working to reduce our dependence on fossil fuels like oil and natural gas. Nuclear energy is an ideal replacement.
Leaders from the US and 21 other countries recently committed to TRIPLING the world’s nuclear energy capacities. This aggressive transition could mean tremendous profits for nuclear-related stocks – and investors who get in on the action early enough.
Our urgent report, Atomic Opportunity: Nuclear Energy's Comeback, explores the key players and technologies driving this opportunity, including 3 standout stocks poised to benefit the most.
Download Atomic Opportunity: Nuclear Energy's Comeback free today.
Want the latest recommendations from Zacks Investment Research? Today, you can download 5 Stocks Set to Double. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
PTC Therapeutics, Inc. (PTCT) : Free Stock Analysis Report
Olema Pharmaceuticals, Inc. (OLMA) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.